By Rich
Haridy
July 12, 2022
Facebook
Twitter
Flipboard
LinkedIn
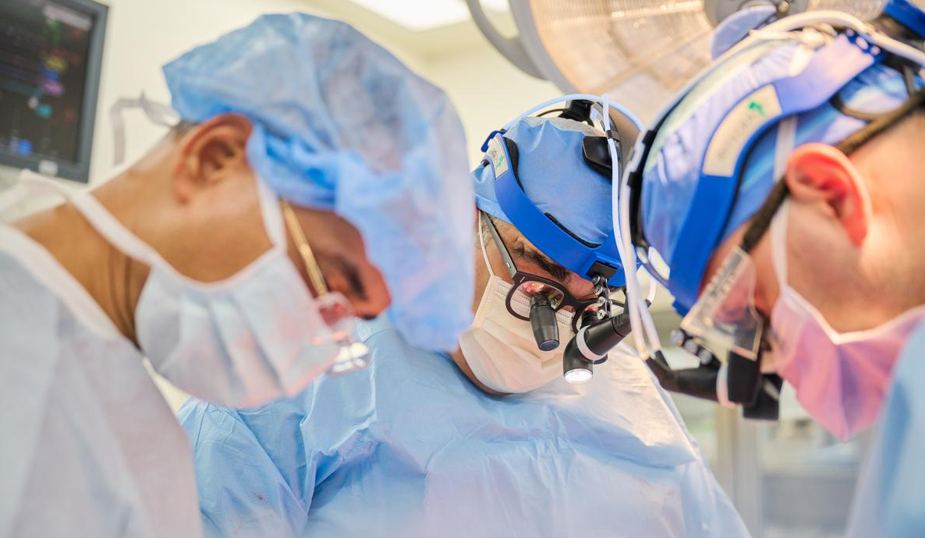
Nader Moazami, (center) surgical
director of heart transplantation at the NYU Langone Transplant Institute,
leads the team as a genetically modified pig heart is transplanted into a
recently deceased donor on July 6, 2022, in New York Photo credit: Joe Carrotta for NYU Langone Health
VIEW 1 IMAGES
This year is certainly turning into a significant landmark
for the field of xenotransplantation. For decades,
scientists have been working to solve the organ shortage crisis. One strategy
has been to develop
genetically modified pigs with organs designed to not be rejected when
transplanted into human bodies.
Earlier this year an extraordinary procedure by surgeons at
the University of Maryland Medical Center completed
the first pig-to-human heart transplant. Through a compassionate
use provision from the US Food and Drug Administration (FDA) the pig heart was
transplanted into a live human, who subsequently
lived for two months before passing away from heart failure.
That xenotransplantation procedure into a live human was an
unexpected jump forward for the field. Before that, researchers at NYU Langone
were at the front of the pack with their work testing pig organs in recently
deceased human subjects.
Last year, the NYU team were the first group in the world to
transplant genetically modified pig organs into humans. Across two procedures,
surgeons successfully transplanted pig kidneys into recently deceased subjects.
The novel paradigm has been referred to as
“whole-body donation,” and it involves volunteers offering their entire body to
science for studies that keep them on life-support for several days after brain
death. Robert Montgomery, leading the research from the NYU Langone Transplant
Institute, says this work is a crucial stepping stone for the field of
xenotransplantation, and before this point these organs had only been tested on
non-human primates.
“Our greater purpose is to address the organ shortage and
provide another option for the more than 100,000 people nationwide waiting on
that lifesaving gift,” Montgomery explained. “The paradigm of whole-body
donation – when organ donation is not a viable option – is critical to this
work moving forward. We are so grateful to the families who volunteer to
participate in this research, which will lead to saving untold thousands of
more lives.”
The two new procedures were completed over the past six
weeks. Both human donors were kept alive on ventilator support for 72 hours
after being declared brain dead.
The pig hearts transplanted into the donors had been
engineered with 10 specific genetic modifications. Six of those modifications
were to include “human transgenes” and four modifications were to knock out
certain pig genes that can promote organ rejection.
The protocol for the procedures specifically attempted to
follow current clinical heart transplantation standards. So only standard
immunosuppressive drugs were used, unlike the Maryland pig-to-human
xenotransplant which used certain experimental medications. And standard
storage and transportation methods were deployed.
“Our goal is to integrate the practices used in a typical,
everyday heart transplant, only with a nonhuman organ that will function
normally without additional aid from untested devices or medicines,” said Nader
Moazami, surgical director on the project. “We seek to confirm that clinical
trials can move ahead using this new supply of organs with the tried-and-true
transplant practices we have perfected at the NYU Langone Transplant
Institute.”
The experimental procedures were deemed a success, with no
signs of rejection as the organs functioned perfectly normally across the
72-hour follow-up. Speaking to CNN Moazami said three days was decided to
be the most ethical timeframe to explore the efficacy of these procedures but a
lot more work will be needed before this kind of xenotransplantation becomes a
clinical reality.
"There's still a long way to go before we go from here
to clinical transplantation to support a patient in the longer term," he
added. "There's still many, many, many questions that need to be
answered."
Source: NYU Langone Health