By Michael
Irving
June 07, 2022
Facebook
Twitter
Flipboard
LinkedIn

A puddle of the liquid gallium and platinum
catalyst used in the new study Photo credit: Dr Md. Arifur Rahim, UNSW Sydney
Platinum
is a powerful catalyst for many different reactions, most commonly used
in fuel cells and in catalytic converters to
clean up vehicle emissions. The problem, however, is that it’s one of the
rarest and most expensive metals on Earth, limiting the scale of devices with
platinum catalysts. As such, scientists have been experimenting with new ways
to reduce the amount of
platinum needed for these devices or even remove it entirely.
Now,
researchers in Australia have found a novel way to drastically reduce the
amount of platinum required for these reactions. Rather than anchoring platinum
atoms onto a solid matrix, the team found a way to suspend them in a liquid,
making it more efficient and reusable.
First,
platinum is dissolved into gallium at around 300 °C (572 °F) for a couple of
hours. Then, once the mixture cools down, it becomes a catalytic material that
remains liquid above gallium’s low melting point of 29.8 °C (85.6 °F), which is
room temperature on a warm day. That’s far lower than platinum’s usual melting
point of 1,768 °C (3,215 °F).
The
resulting material had some amazing properties. It could perform both oxidation
and reduction reactions, where oxygen is added to or removed from a substance.
Even with platinum making up just 0.0001 percent of the atoms in the alloy, the
liquid catalyst was over a thousand times more efficient than a solid catalyst
composed of 10 percent platinum. Better yet, by-products don’t build up on the
catalyst and reduce its effects like they do for solid ones.
On
closer inspection of the liquid catalyst, the team found that no two atoms of
platinum ever touch each other – they remain dispersed throughout the gallium.
Strangely, the platinum seems to be exerting its influence on the surrounding
gallium, which does the catalytic work instead.
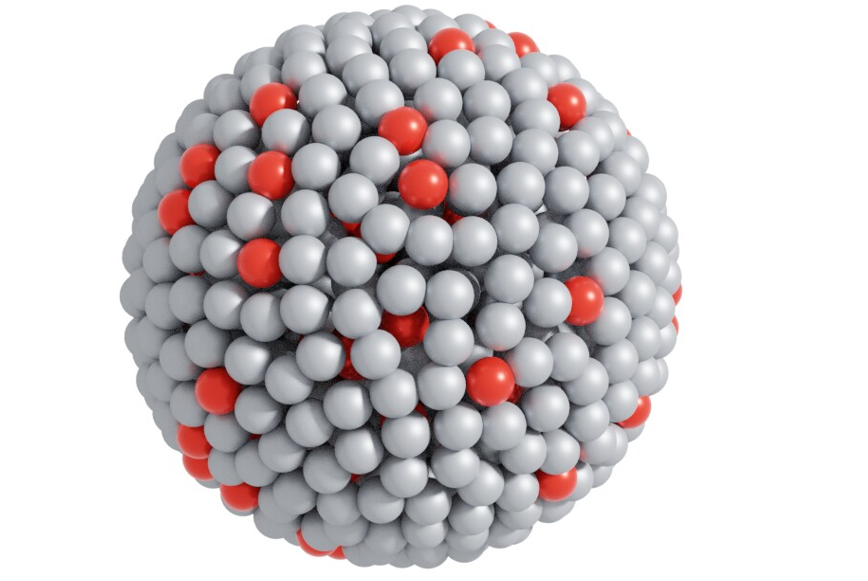
An
atomic model of the liquid metal catalyst – red balls represent platinum atoms,
while gray balls are gallium Photo credit:
Dr Md. Arifur Rahim, UNSW Sydney
“The
platinum is actually a little bit below the surface and it’s activating the
gallium atoms around it,” said Dr. Andrew Christofferson, an author of the
study. “So the magic is happening on the gallium under the influence of
platinum. But without the platinum there, it doesn’t happen. This is completely
different from any other catalysis anyone has shown, that I’m aware of.”
The
team says the new technique demonstrates a way to stretch our thin supplies of
platinum further, allowing far smaller amounts to be used for the same or even
stronger catalytic effects. This should also bring costs down, allowing
effective catalysts to be used more widely.
But
the benefits go beyond platinum – the team says this kind of liquid metal
catalyst could be tested with over 1,000 other possible combinations of
elements, resulting in just as many different reactions. Investigating these
will be a focus of future work.
The research was published in the journal Nature Chemistry.
Source: Scimex